The Nobel Prize in Physiology or Medicine was awarded to Americans Victor Ambrose of the University of Massachusetts and Gary Ravkan of Harvard Medical School “for their discovery of microRNA and its role in post-transcriptional gene regulation.”
The information stored in chromosomes can be compared to instructions for all the cells in the body. Each cell contains the same set of genes and the same set of instructions. However, different types of cells, particularly muscle and nerve cells, are quite different from each other and perform different functions. These differences are ensured by multi-level gene regulation, due to which each type of cell has its own set of genes active and the necessary instructions. The laureates discovered one of the mechanisms of gene regulation, where very short RNA molecules called microRNA play a key role. The more complex the organism, the more types of microRNA it has: for example, about 2,000 have already been discovered in humans. The laureates’ discovery has not yet been used in medicine, but it may prove useful in the treatment of cancer.
Although microRNAs are encoded in a small part of the genome, under normal physiological conditions they perform a number of key functions in development, cell differentiation, regulation of gene expression, the cell cycle and apoptosis, writes the journal Biomolecule.
These small regulators are an extremely important tool for the body. A disruption in the work of one or a small subset of microRNAs has a profound effect on the expression patterns of hundreds of mRNAs. And this, in turn, like a chain, entails disruptions in the fundamental biological processes of the cell. The result of all these changes is often a tumor. Thus, in an instant, microRNAs turned from important controllers of the cell cycle into saboteurs for their own body. This behavior is typical of oncogenic microRNAs.
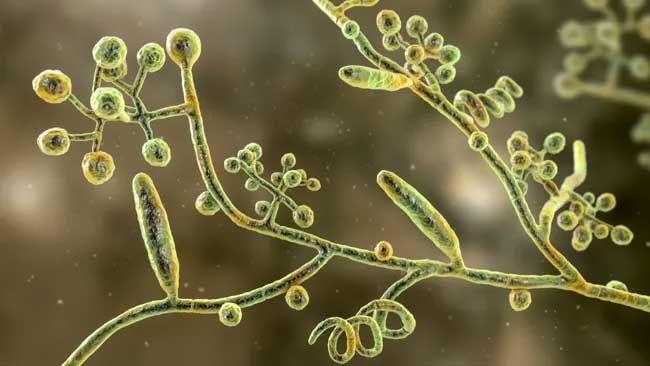
Self-assembled RNA triple helix hydrogel scaffold for modulating microRNAs in the tumor microenvironment. Cryosection of the dendrimer-dextran adhesive hydrogel (12 μm thick) showing the morphology of the adhesive (dextran aldehyde was labeled with Alexa Fluor 405). Red spots represent triple helix nanoparticles containing the oligonucleotides Q705 (red) and Q570 (green).
But there are also oncosuppressor microRNAs, which, as if trying to rehabilitate themselves, inhibit tumor formation processes and promote cancer remission. This division is quite ambiguous, since some microRNAs can be oncosuppressor in some conditions, and oncogenic in others.
Given these opposing functions, it is difficult to draw firm conclusions about the role of microRNAs in carcinogenesis, but numerous studies have detailed their effects on the cell cycle, metastasis, and angiogenesis in the context of cancer development.
Not every disease can boast such resonance in society as cancer. This collective term covers a wide group of diseases that can be called a “global problem” without exaggeration. The issue of cancer treatment remains relevant to this day. According to WHO, in 2020, this disease claimed about 10 million lives, which corresponds to about one sixth of all deaths.
MicroRNAs (miRNAs) are a class of small non-coding RNA molecules, sequences with an average length of 20 nucleotides. They are typically assembled in the nucleus following two main pathways: canonical and non-canonical.
The first involves transcription of primary microRNA (pri-miRNA) by RNA polymerases II or III. This pri-miRNA is processed into a microRNA precursor (pre-miRNA) by a ribonuclease called Drosha. As a result, it is exported from the nucleus to the cytosol, where an endonuclease of the RNase III (Dicer) family converts pre-miRNA into a microRNA duplex (Figure 1).
The maturation of microRNA is completed by the formation of a ribonucleoprotein complex — miRISC (microRNA-induced silencing complex). In the cytoplasm, microRNA associates with the proteins TRBP (transactivation response RNA-binding protein), PACT (protein kinase R activating protein) and one of the proteins of the Argonaute family, resulting in the formation of the RISC complex. Only after the formation of this complex does microRNA acquire functional activity. The other chain of microRNA (passenger) is often (but not always) removed.
The biogenesis of most microRNAs occurs by this mechanism, but this is not the only pathway. About 40% of animal microRNAs are encoded in introns of protein-coding genes, and therefore many pri-microRNA transcripts are both pre-mRNA and pri-microRNA (Cai et al., 2004; Kim et al., 2009). Such introns, called myrtrons, are spliced to form microRNA hairpins suitable for Dicer cleavage. In this case, the microRNA bypasses some steps of the canonical pathway.
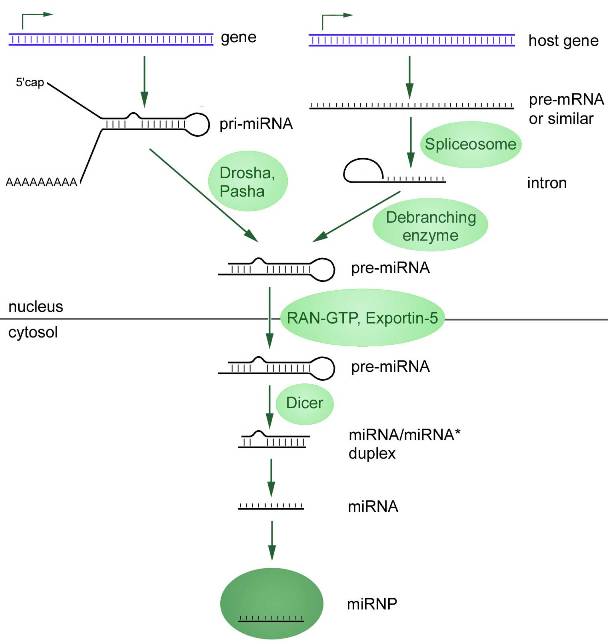
Figure 1. Two pathways of microRNA biogenesis, starting in the cell nucleus with transcription. The canonical pathway is shown on the left: pri-miRNA undergoes 5′-capping and often 3′-polyadenylation (except for genes adjacent to Alu repeats). With the help of Drosha Pasha enzymes, pri-miRNA is trimmed, transforming it into a precursor miRNA (pre-miRNA) with a shorter sequence. Non-canonical pathway: primary microRNA (pri-miRNA) is transcribed from introns (myrtrons) in the nucleus, which is then spliced by spliceosomes, and then forms a characteristic hairpin. Pre-miRNA formed as a result of both pathways of synthesis is exported from the nucleus to the cytoplasm, where it is transformed into mature miRNA by the Dicer enzyme.
There are many more possible variants of microRNA biogenesis, but these two can be called the main ones.
The role of microRNAs should not be underestimated. They are very important regulators of many biological processes. The fact that about a third of protein-coding genes are controlled by microRNAs clearly indicates the wide range of influence of these molecules, which directly or indirectly affects almost all cellular pathways. The main factor of microRNA influence is the regulation of gene expression by repression, blocking their translation or destruction of specific transcripts of messenger RNA (mRNA).
The regulatory activity of microRNA is closely related to the RISC protein complex, which is responsible for two important stages: target recognition and implementation of the regulatory effect. Recognition of the target mRNA and binding are provided by the complementary interaction of the microRNA recognition site and the recognition site (miRNA response element, MRE) located in the target mRNA. The regulatory effects of microRNA are provided by many protein components of RISC, such as AGO (Argonaute), which, after recognition and binding to the target, initiates the process of developing the effect on the target.
The RISC protein complex is a very important structure that takes part in many processes of gene activity regulation (Figure 2A).
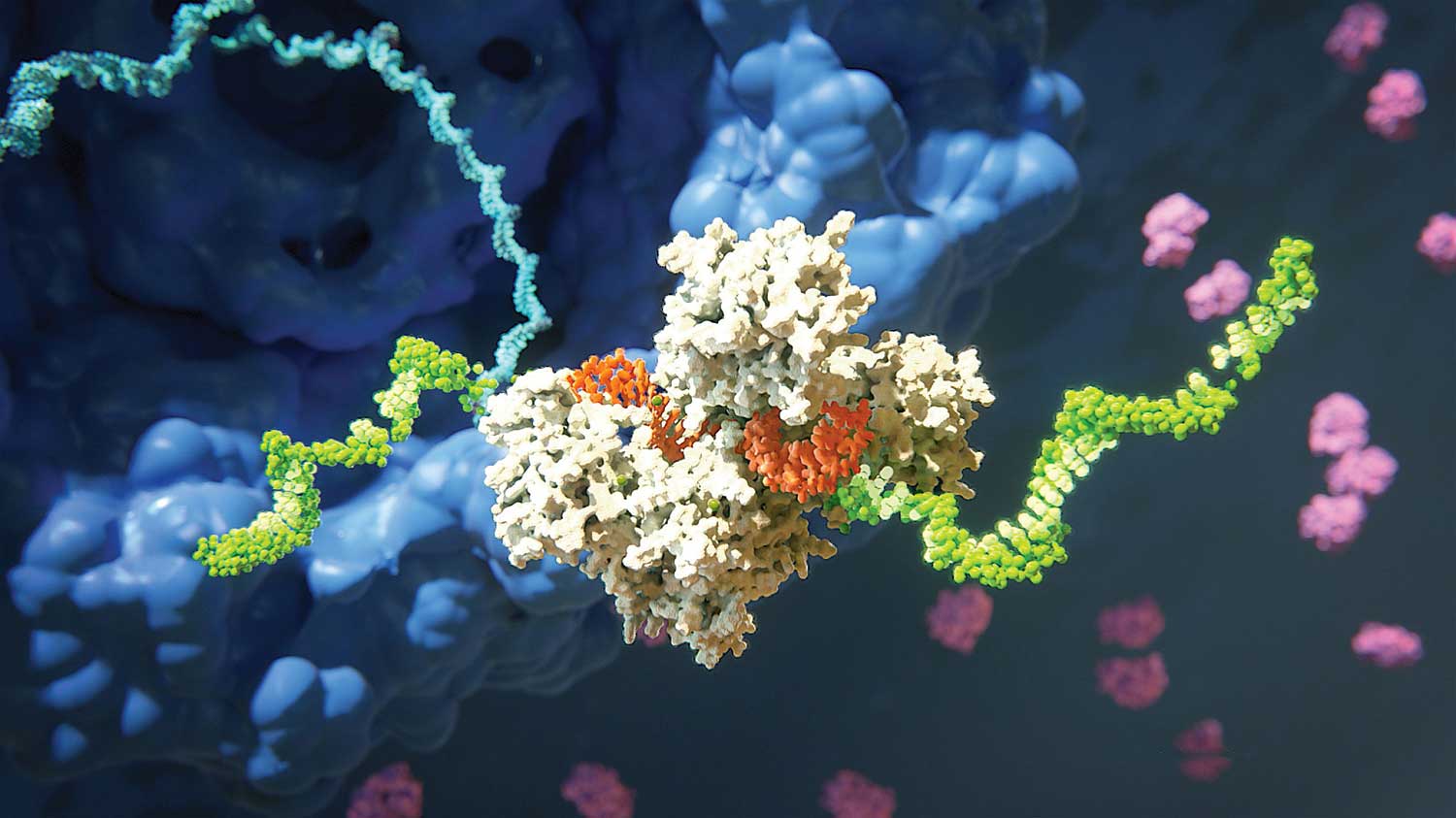
Figure 2A. The RNA-induced complex (RISC) that cleaves a target mRNA. RISC (shown here in white) includes the protein Argonaute and a single-stranded RNA molecule (orange) derived from small interfering RNA (siRNA). It cleaves the mRNA (green) that is complementary to one of the strands of the miRNA. This process ends with the degradation of the target mRNA.
The interaction of microRNA with the RISC protein complex occurs according to the following scenario: one of the microRNA duplex chains (the leading one) is introduced into the protein complex, forming a structure that binds the 3′-untranslated end (3′ UTR) of the target mRNA. After this, the microRNA acts on its target, which can result in the destruction of mRNA by nucleases or the suppression of its translation (Figure 2B).
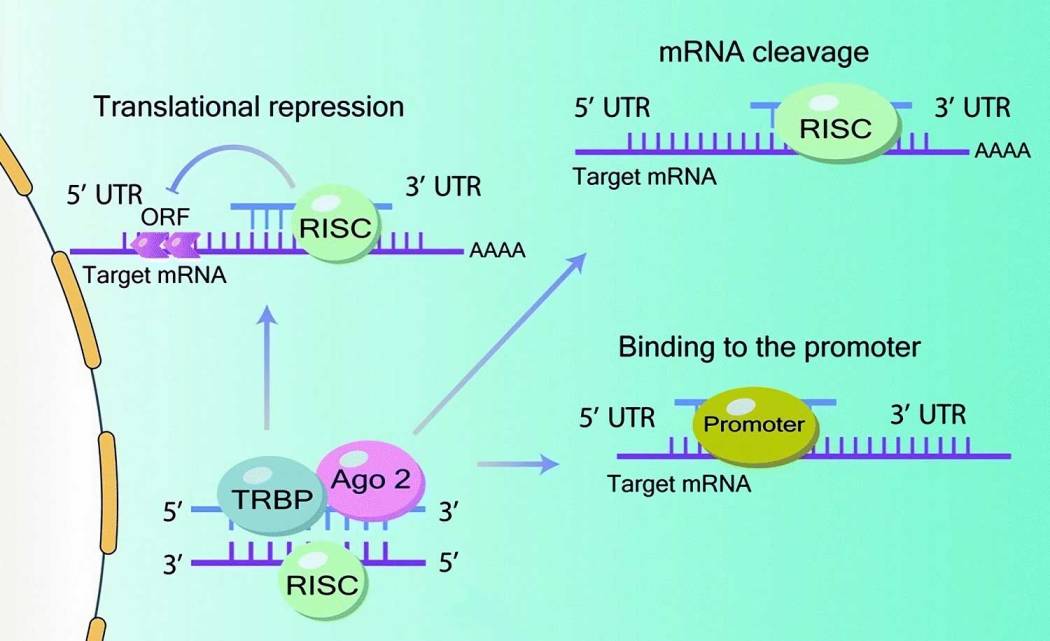
Figure 2B. Mechanism of action of microRNA: After pre-microRNA is exported from the nucleus (via Ran-GTP-dependent exportin-5) to the cytoplasm and processed to mature microRNA, the RISC complex is assembled by interaction of TAR RNA-binding protein (TRBP) and Argonaute 2 (Ago 2) to target the 3′-untranslated regions (3′-UTR) of target mRNAs. Translation is then inhibited by complementary binding to the 3′-untranslated end (3′ UTR) of the target mRNA. However, other interactions are also possible: degradation of mRNA or inhibition of translation due to binding to the promoter of the coding region and 5′ UTR.
In general, it is believed that animal microRNAs function overwhelmingly by repressing translation, while plant microRNAs function by post-transcriptional suppression of gene expression. At the same time, microRNAs are able to influence translation at the initiation and elongation stages. Interestingly, in addition to transcription suppression, other mechanisms of microRNA action have been discovered. Thus, microRNAs can stabilize mRNAs and enhance their translation, act as “bait” by preventing the interaction of protein factors with their RNA targets, and participate in the maturation of other microRNAs.