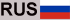In protoplanetary disks, water is virtually omnipresent. Recent studies of the water content of early planetary systems like ours show that water is an abundant and ubiquitous molecule, originally synthesized on the surface of tiny grains of interstellar dust by hydrogenation of frozen oxygen, reports the journal Elements. In the molecular cloud from which a new planetary system will emerge, oxygen attaches and freezes to the dust grains it encounters. Once a hydrogen molecule intersects with this frozen oxygen, water ice is formed.
The reaction can produce two types of water: ordinary water, consisting of oxygen and hydrogen, or heavy water, consisting of oxygen and deuterium – an isotope of hydrogen with a proton and neutron in the nucleus. The formation of water ice around the dust grains is the first stage of a process the researchers call the “cold phase.” Over time, matter accumulates in the center of the nebula, and gravitational force begins to act. As temperatures rise around the center—an area experts call a hot corino—the ice turns into water vapor. Water becomes the most abundant molecule in this area.
“A typical hot corina contains about 10,000 times more water than Earth’s oceans,” write the two authors, Cecilia Ceccarelli, an Italian astronomer at the Institute of Planetary Sciences and Astrophysics in Grenoble, France, and Fujun Du, an astronomer at the Purple Mountain Observatory. in Nanjing, China. This stage of ice sublimation into vapor is the second phase of the process, which researchers call the “protostar phase.”
This protostar has not yet begun its fusion reaction, but it is beginning to rotate; the gas and dust surrounding it form the so-called protoplanetary disk, from which various objects of this new system will originate. The young star continues to gain mass but produces little heat; the protoplanetary disk is still cold.
Then the third phase of the process begins: the water vapor formed in the second phase recondenses in the coldest parts of the protoplanetary disk; The dust grains are again covered with icy mantles. From these dust grains, surrounded by frozen water, a planetary system gradually forms: planets, comets and asteroids appear that orbit their star. This is how the Earth came into being.
Therefore, water synthesis occurs at two points in time, under different conditions: the first time, when the system is still a cold cloud, the second time, when a protoplanetary disk is being formed. In our solar system, the age of water formed as a result of the first synthesis is 4.5 billion years. In order to determine the age of Earth’s water, it is necessary to determine how much of this water ended up on Earth.
The researchers note that the ratio of hydrogen isotopes is critical to understanding the origin of water in planetary bodies. During the cold phase, the temperature is extremely low, leading to a phenomenon called superdeuteration. Under these conditions, more deuterium enters the water ice.

At the moment of the birth of the Universe, a few seconds after the Big Bang, the amount of deuterium was very small – just one atom of deuterium for every 100,000 atoms of standard hydrogen (or protium). But in hot corina, because of the superdeuteration that precedes it, the abundance does not follow this pattern. “In hot korynes, the HDO/H2O ratio is only slightly less than 1:100. [The abundance of doubly deuterated water, D2O, is 1/1000 that of H2O, or about 107 times greater than would be predicted from the D element abundance ratio /H],” say the study authors.
The researchers showed that the abundance of heavy water is a characteristic of the first synthesis. To find out how much of this water reached Earth, they compared values for the ratio of heavy water to normal water, HDO/H2O, on Earth with values at Corino hotspots in other emerging systems. Previous studies have shown that this ratio is about ten times higher than the elementary D/H ratio in the Universe and, therefore, at the birth of the Solar System.
Researchers estimate that between 1% and 50% of Earth’s water comes from the early phase of the solar system’s birth, so much of our water is 4.5 billion years old. This water is “likely inherited” from planetesimals, not comets.
Deuterium in nuclear energy
Deuterium is a heavy isotope of hydrogen. There is negligible amount of deuterium in drinking water – about 150 atoms per 1 million hydrogen atoms. Deuterium water, also known as heavy water, is used in the nuclear industry as a fast neutron moderator and coolant. Deuterium is isolated in several ways: using electrolysis, rectification of liquid hydrogen or hydrogen-containing compounds. Entire factories are involved in the production of heavy water, which, in order to obtain a high concentration of deuterium, evaporate hundreds of tons of water under certain conditions. To obtain toxic heavy water, deuterium must be concentrated several thousand times.
Japan received the “first plasma” at the world’s largest thermonuclear reactor JT-60SA, these experiments at JT-60SA will better prepare for the launch of the reactor in France. At subsequent stages, the paths of these reactors will diverge. The Japanese reactor can only operate on deuterium fuel, while the ITER reactor will eventually be able to switch to more efficient deuterium-tritium fuel. However, experiments on JT-60SA will allow the Japanese to develop their own fusion power plant, Project DEMO, which they intend to build by 2050. In the meantime, the industry is set by the Chinese, whose experimental thermonuclear reactors heat plasma to temperatures above 100 million °C for hundreds of seconds.
In many countries of the world today, research and experiments are being conducted to put thermonuclear fusion into the service of energy and to develop a model of an industrial thermonuclear reactor. Unfortunately, these plans run into many technical and scientific problems. One of them is the absorption of hydrogen by the inner wall of the reactor. For the first time in the world, the National Research University MEPhI has developed a mathematical model designed to help solve this problem.
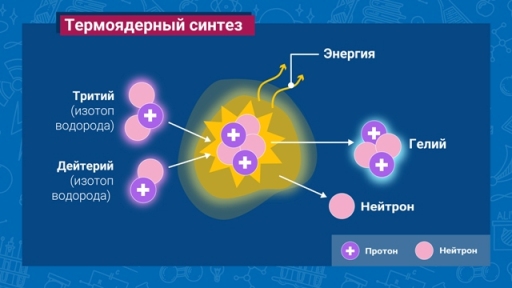
The heart of a thermonuclear reactor is a chamber in which hot plasma burns: the process of synthesis of helium atoms from hydrogen atoms occurs. Magnetic fields prevent the burning plasma from coming into contact with the surface of the chamber, but, unfortunately, it is impossible to completely eliminate the interaction of the plasma and the reactor walls.
Tiny “sprays” of hot plasma can hit the wall, knocking out small fragments and particles – sometimes literally single atoms. These particles that break off from the wall first fly around the reactor chamber for some time and then settle back onto the wall. The problem, however, is not even this, but that the particles split off from the surface, settling, carry with them atoms of the working gas of the plasma – and these are various isotopes of hydrogen.
This process is called co-deposition – that is, the joint deposition of tungsten and hydrogen particles onto the surface. As a result, a hydrogen-saturated film grows on the surface of the thermonuclear installation chamber. It is this mechanism that is responsible for most of the hydrogen accumulation in the chamber.
This is bad for three reasons. Firstly, the working gas in a thermonuclear reactor contains a radioactive isotope of hydrogen – tritium. Its accumulation in the reactor wall can create radiation safety hazards. Secondly, tritium is a very expensive raw material, and if it is lost for the working process, this negatively affects the economics of the fusion reactor.
Thirdly, at the moments of plasma discharges, the surface will heat up and the absorbed hydrogen will be released back – and this will already affect the thermonuclear reaction itself. Any hydrogen that will be released from the reactor wall will be cold compared to the plasma. The energy of the plasma in the reactor, measured in electric volts, will be approximately 100,000 times greater than the energy of the hydrogen released from the wall. And cold hydrogen can negatively affect plasma combustion.
The most important issue that is being solved today within the framework of the international thermonuclear reactor project ITER is to predict the accumulation of hydrogen in the walls of the reactor – and this, in turn, is necessary, for example, in order to know how often it will be necessary to “disinfect” the walls from accumulated hydrogen. Senior researcher at the Department of Plasma Physics of the National Research Nuclear University “MEPhI” Stepan Krat became the author of the world’s first mathematical model of the process of hydrogen accumulation in the surface of a thermonuclear reactor.

One of the important points of the proposed theory is that it examines the behavior of different isotopes of hydrogen in this process. The proposed power fusion reactors (including ITER) will use a mixture of two isotopes of hydrogen – deuterium and tritium. The specific gravity of these two gases is different (tritium is heavier). Stepan Krat and his colleagues were the first to hypothesize that the parameters of the coprecipitation processes of deuterium and tritium will differ from each other, and the situation for a mixture of these two gases will obey the third set of parameters.
The proposed theoretical model describes the case for a mixture of two or more hydrogen isotopes and shows that the mechanisms of their absorption by the reactor walls obey complex nonlinear patterns, depending on many factors. The simulation results were published in the Journal of Nuclear Materials (link is external). In addition to Stepan Krat, co-authors of the publication were employees of the National Research Nuclear University MEPhI Yuri Gasparyan and Alexander Prishvitsyn. After publication, Stepan Krat’s hypothesis was experimentally tested at the National Research Nuclear University MEPhI on a mixture of two non-radioactive isotopes of hydrogen – deuterium and protium.